Abstract
After hematopoietic allogeneic stem cell transplantation (HCT), B cells reconstitute in the presence of alloantigen in a nucleic acid (NA)-rich environment. Ongoing tissue and cellular damage results in release of endogenous NA that may serve as ligand for endosomal Toll-Like Receptors (TLRs). Alloantibodies and autoantibodies, including NA-binding antibodies, have been associated with the presence of cGVHD manifestations. In patients after HCT, B cells are constitutively activated via key proximal B Cell Receptor (BCR) signaling molecules, Syk and BLNK (Allen JL et al Blood 2014, Flynn R et al Blood 2015) . In mouse models of autoimmunity, TLR7 or TLR9 can operate in conjunction with the BCR to mediate autoantibody production (Suthers A et al. Front Immunol. 2017). Whether TLR7/9-BCR signaling contributes to cGVHD remains unknown.
We studied responses by TLR9 and TLR7 in B cells from patients >12 months post-HCT who had active, inactive or no cGVHD at the time of sample acquisition and were not receiving high dose steroid. In contrast to a previous study (She K et al. BBMT 2007) and consistent with findings by others (de Masson A et al Blood 2015), we did not find a consistent increase in B cell response to TLR9 agonist. Instead we found significantly higher TLR7 transcript expression in B cells from patients with active cGVHD (n=7) compared to no/inactive cGVHD patient B cells (n=11, p=0.042). TLR7 over-expression in mice leads to anti-RNA antibody production by immature B cells (Giltiay NV et al JEM 2013). We employed ELISA to measure antibodies to a known RNA-containing autoantigen, Ro-52 in a group of 66 plasma samples. We found that significantly more patients with active cGVHD (n=32) had anti-Ro-52 antibody compared to inactive cGVHD patients (n=22) or patients without cGVHD (n=12) (Fisher exact test for both comparisons = p<0.0001). This suggests potential opposing roles for TLR9 and TLR7 in cGVHD (Sharma et al, J.Immunol. 2015), and allowed us to hypothesize that B cells in cGVHD were aberrantly activated via TLR7.
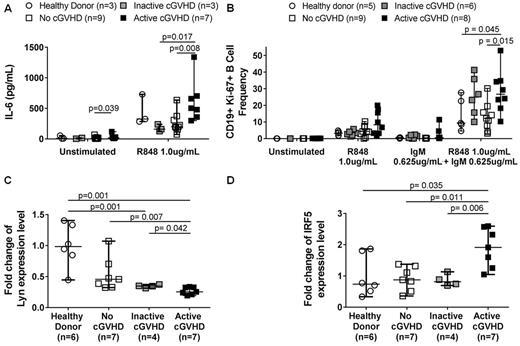
To determine if TLR7 activation was increased in cGVHD B cells, we measured IL-6 production. Without ex vivo stimulation, cGVHD patient B cells produced significantly more IL-6 (p=0.039) (Fig.1A). After TLR7 stimulation with R848, B cells from patients with active cGVHD had significantly increased IL-6 production compared those from patients without (p= 0.008) or those with inactive cGVHD (p=0.017) (Fig.1A). We next investigated the influence of the BCR-activated state in cGVHD on TLR7 responses and signaling. Using flow cytometry, we measured Ki-67 expression as a marker of activation after ex vivo stimulation with both low level surrogate BCR antigen and R848. We found that B cells from active cGVHD patients were significantly more responsive compared to no cGVHD patients (p=0.015) and healthy donors (p=0.045), revealing increased synergistic BCR-TLR7 signaling in cGVHD B cells (Fig.1B). To further investigate this, we employed qPCR to measure levels of the proximal BCR signaling molecule, Lyn, known to negatively regulate anti-NA antibody production (Lamagna et al. J. Immunol. 2014). We found that Lyn expression was significantly decreased in B cells from active cGVHD patients compared to healthy donor (p=0.001), inactive (p=0.042) or no (p=0.007) cGVHD patients (Fig.1C). Lyn can also negatively regulate TLR7/9 activation by directly associating with Interferon Regulatory Factor 5 (IRF5), which is important for pro-inflammatory cytokine production, including IL-6 (Ban et al, J.Immunol. 2016). Consistent with a role for Lyn in TLR7 activation, we found that IRF5 was significantly increased in active cGVHD patient B cells compared to all other groups (healthy donor, p=0.035; inactive cGVHD, p=0.011; no cGVHD, p=0.006) (Fig.1D). Notably, no difference in expression was found in downstream TLR7 transcription factors, IRF3 and IRF7 . Using a mouse model (Zhang C et al Blood 2006), we found significantly higher IRF5 expression in splenic B cells in animals with cGVHD manifestations (p=0.005). Ongoing mouse work will ascertain mechanistic roles for Lyn and IRF5 in TLR7 signaling by cGVHD B cells. Together data support a potentially pathogenic role of TLR7 signaling in cGVHD B cells. Development of agents that block this newly elucidated TLR7-BCR signaling axis in cGVHD is warranted.
This work was supported by National Institutes of Health grant R01 HL129061
Rizzieri: Erytech: Research Funding; Shire: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal